Suncombe, formed in 1961, design and manufacture high quality critical automatic processing systems for the pharmaceutical, biotechnology and research sectors. We are experts in BioWaste Effluent Treatment, Cleaning In Place, GMPWashers, GMP Vessels, Sterile Liquid Processing Skids/Modules and Custom Production, Storage, and Distribution Solutions for validation and compliance to cGMP requirements.
Trusted by 17 of the Top 20 Biopharma Companies Worldwide, You’re In Good Company
All Suncombe equipment has been redeveloped for sustainability purposes and incorporate techniques and technologies to minimise its impact on the environment. Some of these features include minimizing the volumes, thereby decreasing water and effluent generation and chemical usage, more efficient motors, more optimised spray devices and ultra-configurable cycles.
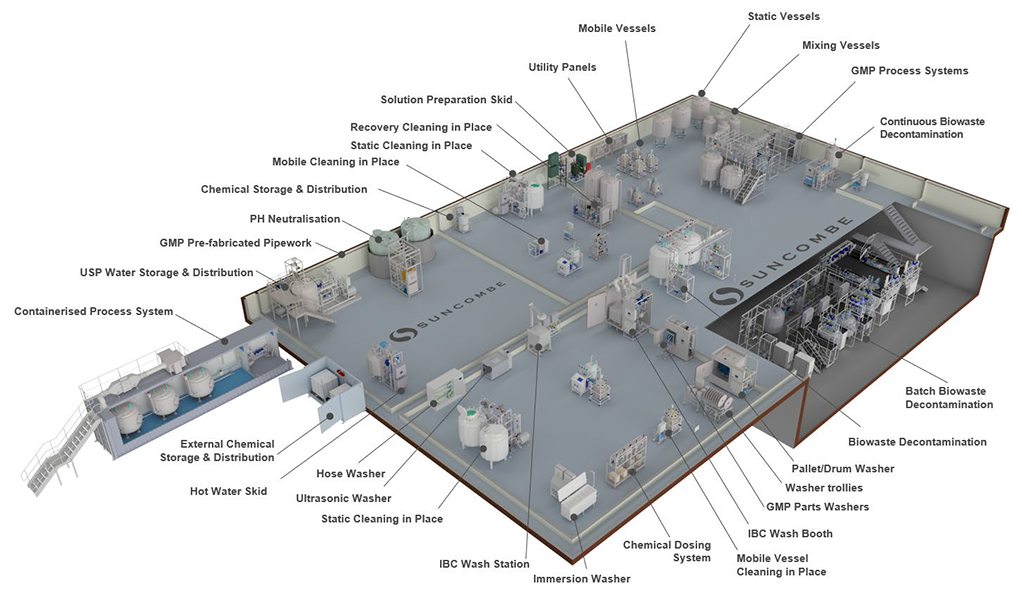
Biowaste Decontamination Systems
Cleaning In Place
Washers
Tanks &
Vessels
Skids &
Systems
Automation
“We are proud to have delivered most of the category 4 Effluent Decontamination Systems in the United Kingdom.”
Dave Adams, Director, Suncombe.
DOWNLOAD OUR BROCHURE
Our Commitments
Our commitment is to Design and Build in-house all aspects of our equipment including software, electrical and mechanical. This commitment has produced a work force made up of highly motivated and experienced personnel who have all proved their dedication to the industry.
Our Values
To act with integrity and show respect, we are passionate about our business and our products and aim for Engineering Excellence at all times. We offer Value for Money whilst priding ourselves on Innovative Design. We use robust Health and Safety guidelines in addition to the promoting environmentally responsible practices.
Our Expertise
Our company was formed in 1961 specifically to supply a high-quality range of CIP, effluent treatment and sanitary processing equipment. To continually achieve our goals, we use the most up-to-date facilities and employ the best-qualified and experienced staff.




Why use Suncombe
- The oldest and most experienced independent UK manufacturer of BioWaste Decontamination and Cleaning In Place equipment
- Risk Aversion or De-risking – Suncombe takes away your Risks – Suncombe takes away your risks by ensuring that our equipment is matched to comply with your requirements. Our vast experience allows us to apply leverage to countless similar projects throughout the world, to ensure your project meets all requirements and legislation. With countless systems operating throughout the world, every individual project is given the same thorough treatment, by the very experienced Suncombe organisation.
- Robustness and Longevity – designed for 25 year + life – which means lower cost of ownership and minimal maintenance downtime
- Reliable and Repeatable Results – guarantee of repeatable process on all Suncombe equipment, so product quality is maintained, and contamination risk is virtually eliminated
- Versatility – fully configurable recipes for every process – maximise the utilisation of your Suncombe equipment
- Useability – simple intuitive user interface for operation, with user levels accessed for more complex tasks – your staff will quickly be up to speed
- Validateability – suitable for validation and verification – reassuring you that you have a secure audit trail
- Reports – batch reports and process reports, electronic or paper based – ideal for monitoring issues and spotting trends
- Online Connection – on line connection to Suncombe After Care department for off-site support, so that any problems can be quickly diagnosed and solved
“Suncombe is the oldest independent UK manufacturer of BioWaste Decontamination and Cleaning In Place equipment and has been since 1961. We are all proud of our heritage and approach the future with passion, integrity and a drive for excellence in everything that we do.”
Dave Adams, Director, Suncombe.
Speak to one of our specialists
Our company was formed in 1961 specifically to provide state-of-the-art equipment and systems for the critical engineering, high purity and GMP sectors.
Life Science
Life Science
Medical and research
Medical and research
Critical processing
Critical processing
Custom Manufacturing
Custom Manufacturing
Single Use Technology (SUT)
Single Use Technology (SUT)
Original Equipment Manufacturers (OEMs)
Original Equipment Manufacturers (OEMs)
Our Values
- We act with integrity and show respect
- We are passionate about our business and our products
- We aim for Engineering Excellence
- We offer Value for Money
- We pride ourselves on Innovative Design
- We offer Quality in everything that we do
- We use robust Health and Safety guidelines
- We strive to continuously increase our Knowledge
- We promote environmentally responsible practices






