Static CIP
Introduction To Static CIP
Cleaning In Place (CIP) systems, are defined as equipment and techniques to allow cleaning of process equipment without dismantling or manual cleaning with minimal operator involvement.
We are exceptionally proud at Suncombe knowing that our founder pioneered the technology of CIP in the 1950’s. Suncombe CIP Systems have been supplied for over 60 years and are used throughout the world in many different industries and sectors. This has given us a wealth of experience in design and manufacture enabling us to produce the most effective and efficient solutions for our clientele.
Product Range
We offer multiple CIP variants to suit our client’s requirements, each offering unique and key benefits:
PureCIP – Our ASME BPE Specification build for full validation, utilised worldwide by the biopharmaceutical industry. Read more>
CIPOne – Utilising standard pre-approved modules, the CIPOne variants is a fully modularised system that offers an economic solution to CIP whilst maintaining the quality of build and operation that Suncombe clients expect entry. Read more>
CIP+Plus – Our Sanitary specification build, used throughout the world in the Dairy, Food, Beverage, Cosmetics, Personal Care and other Processing Industries. Read more>
Satellite – Specifically developed for clients who have existing CIP tanks and for export markets where CIP tanks are supplied locally.
Product Range
We offer multiple CIP variants to suit our client’s requirements, each offering unique and key benefits:
PureCIP – Our ASME BPE Specification build for full validation, utilised worldwide by the biopharmaceutical industry. Read more>
CIPOne – Utilising standard pre-approved modules, the CIPOne variants is a fully modularised system that offers an economic solution to CIP whilst maintaining the quality of build and operation that Suncombe clients expect entry. Read more>
CIP+Plus – Our Sanitary specification build, used throughout the world in the Dairy, Food, Beverage, Cosmetics, Personal Care and other Processing Industries. Read more>
Satellite – Specifically developed for clients who have existing CIP tanks and for export markets where CIP tanks are supplied locally.
Speak to a static cip specialist
Static CIP: Streamlining Cleaning Processes for Enhanced Efficiency and Product Quality
In industries that require stringent cleanliness and hygiene standards, such as food and beverage, pharmaceuticals, and biotechnology, effective cleaning processes are essential. Static CIP (Clean-In-Place) systems have revolutionized the way equipment and piping systems are cleaned, offering a reliable and efficient method that minimizes manual intervention and ensures optimal cleanliness.
Static CIP systems are designed to clean and sanitize equipment and piping systems in place, without the need for disassembly. This eliminates the time-consuming process of dismantling and reassembling components, reducing downtime and increasing overall operational efficiency. By utilizing automated cleaning cycles, these systems significantly streamline the cleaning process, allowing for continuous production and minimizing disruptions.
One of the primary advantages of static CIP systems is their ability to reach and clean intricate and hard-to-reach areas within equipment and piping systems. They employ a combination of cleaning agents, temperature, and flow rate to remove stubborn residues, contaminants, and biofilms that may accumulate on surfaces. This thorough cleaning ensures the elimination of potential sources of contamination and minimizes the risk of product defects or compromised quality.
Static CIP systems offer precise control over critical cleaning parameters, such as temperature, concentration of cleaning agents, and contact time. This enables manufacturers to customize and optimize the cleaning process according to the specific requirements of their equipment and products. By fine-tuning these parameters, manufacturers can achieve consistent and reliable cleaning results, enhancing product quality and ensuring compliance with regulatory guidelines.
Furthermore, static CIP systems are designed to minimize water and chemical usage, reducing the environmental impact and operating costs associated with cleaning processes. They employ efficient cleaning techniques, such as recirculation, sequential cleaning steps, and targeted spray patterns, which optimize the use of resources. By minimizing water and chemical waste, static CIP systems contribute to sustainable manufacturing practices and support environmental stewardship.
Static CIP systems also incorporate advanced monitoring and control systems to ensure the effectiveness of the cleaning process. They are equipped with sensors, probes, and automated feedback loops that continuously monitor critical parameters during cleaning operations. This real-time monitoring allows for immediate adjustments and corrective actions if any deviations or issues are detected.
Additionally, static CIP systems provide comprehensive data logging and reporting capabilities, enabling manufacturers to maintain detailed records of cleaning cycles for quality control and regulatory compliance purposes.
In conclusion, static CIP systems have transformed the cleaning processes in industries that prioritize cleanliness and hygiene. By offering automated, efficient, and precise cleaning capabilities, these systems enhance operational efficiency, improve product quality, and ensure compliance with stringent regulatory standards.
Implementing static CIP systems allows manufacturers to streamline their cleaning processes, reduce downtime, and optimize their overall production output. Invest in static CIP systems today to unlock the benefits of enhanced efficiency and product quality in your manufacturing operations.
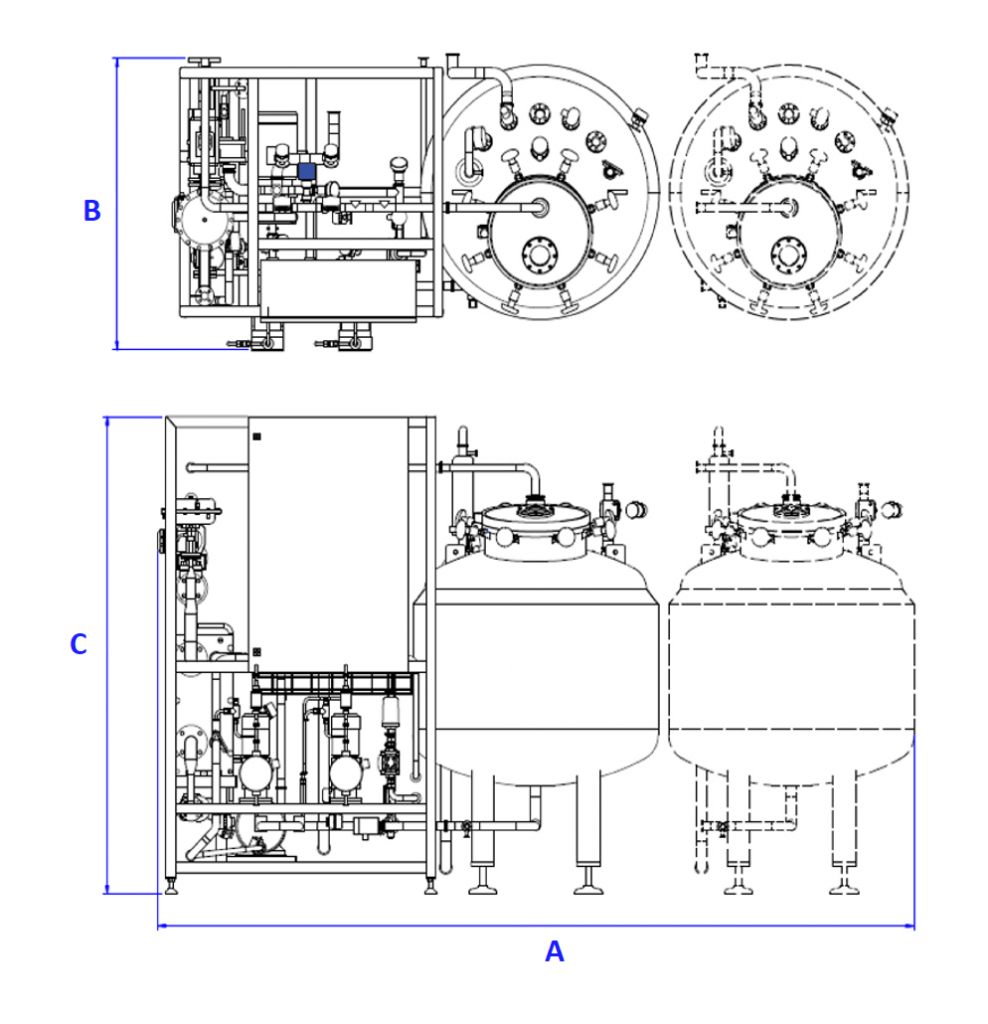
We can offer a range of standardised systems built to a specification to suit your needs. Each of the below CIP Systems can be specified to one of our variants
| Reference | Tank Capacity Litres | Flowrate Litres per minutes | Head Pressure Bar | Dimensions A (mm) | Dimensions B (mm) | Dimensions C (mm) |
|---|---|---|---|---|---|---|
| CIP System™ 600 | 600 | 0-100 | 1-5 | 1,200 | 2,000 | 1,000 |
| CIP System™ 1000 | 1,000 | 0-250 | 1-5 | 2,000 | 2,000 | 1,000 |
| CIP System™ 2200 | 2,000 | 0-500 | 1-5 | 2,500 | 2,000 | 1,250 |
| CIP System™ 3400 | 3,400 | 0-1,000 | 1-5 | 3,000 | 2,000 | 1,500 |
| CIP System™ 5000 | 4,500 | 0-1,250 | 1-5 | 3,000 | 2,000 | 2,000 |
| CIP System™ 10000 | 10,000 | 0-1,250 | 1-5 | 4,000 | 2,000 | 2,200 |
SIP capabilities available in all of our Mobile CIPs. The sterilisation of production equipment carried out by the means of superheated steam.
ATEX versions are available for solvent use and/or aqueous use in zoned areas.
Optional stainless steel covers can be fitted
Pumps and Mixers can be controlled via the4 Hygienic variable speed.
Includes dosing confirmation which can be incorporated within any recipe step at any concentration
Different purities of water from WFI (water from injection to towns water)
Our versatile automation systems allow you to build your own recipes from pre-commissioned steps in any order
All Records and reports will comply to 21CFR11
All vessels are designed PED directive
All our systems can be containerised if required
We have a range of suppliers for each component, therefore prices can be flexible to meet your needs
High quality analytical and process instrumentation
Operator Friendly and intuitive system
We are able to accommodate multiple user requirements, and are experienced in designing and manufacturing systems to various specification variables. To quickly generate an enquiry with us fill in the below form with as much detail as available to you, the form will auto-submit with your initial enquiry. This will enable our experts to begin assessing your system requirements and a sales representative to open a clear and concise channel of communication with you.
If you feel your line of questioning falls outside of our custom form you can contact one of our specialists by:
Phone: +44 (0) 208 804 5555
Email: salesdept@suncombe.com
We are able to accommodate multiple user requirements, and are experienced in designing and manufacturing systems to various specification variables. Quickly fill out your information in the form below so one of our team members can help you with your specific requirements.
We are able to accommodate multiple user requirements, and are experienced in designing and manufacturing systems to various specification variables. To quickly generate an enquiry with us fill in the below form with as much detail as available to you, the form will auto-submit with your initial enquiry. This will enable our experts to begin assessing your system requirements and a sales representative to open a clear and concise channel of communication with you.
If you feel your line of questioning falls outside of our custom form you can contact one of our specialists by:
Phone: +44 (0) 208 804 5555
Email: salesdept@suncombe.com
ASME BPE standard Pipework, fully annealed, chemistry to ASTM A-269, manufactured to ASTM A-270, and 3A Standard.
316 Stainless steel product contact parts, 304 non-contact parts, Hastelloy and Duplex Stainless.
T.I.G Welding; using argon gas purge, using a computer controlled enclosed head orbital welding plant.
PED Vessels, Complete material traceability, PD5500 compliant.
European Hygienic Engineering and Design Group (EHEDG)
Business Practices
- Cost Matrices
- Client Badging
- GAMP
- CE compliant
- Full Document Indexing
- Working to ISPE
- Industry 4.0 aware
- Complete Digital and/or physical Data Manuals
- 3D Modelling
- Corporate member of EBSA
Welding Standards
- BS EN 10204 – 3.1
- BS EN ISO 9606-1:2013
- BS EN ISO 15614-1:2004+A2:2012









Cost Matrices
Client Badging
GAMP
CE Compliant
Full Document Indexing






Working to ISPE
Industry 4.0 Ready
Data Manuals
3D Modelling
Corporate member of EBSA


Welding Standards
- BS EN 10204 – 3.1
- BS EN ISO 9606-1:2013
- BS EN ISO 15614-1:2004+A2:2012




